Trump at Davos
Trump isn’t mad. That would be an easier explanation. In truth he is systematically mendacious, evil to the core, high as a kite on testosterone injections and who knows what other stimulants.
This ramble at Davos was ridiculous, a succession of bluster and lies. Virtually nothing he said was truthful or accurate.
Patriotic Americans need to remove him from office immediately. He is by far the most dangerous man on the planet since Adolf Hitler.
I Trust the BBC Much More than Any Other Media Outlet

Look at who’s attacking it – the Telegraph along with the rest of the press, the Conservatives, Nigel Farage, Reform and Donald Trump. It’s not difficult to know who to trust!
It’s the Daily Telegraph that leads the assault on the BBC and there couldn’t be a sharper contrast between these two news providers. I was brought up seeing my father read the Telegraph every day and I followed him. For most of my adult life it was by far the best newspaper, both for the sheer quantity of news it published and the middle road it took between the Times, which could be very dry and the tabloids, which have always been a trivial form of entertainment rather than serious information. In the last few years, however, it has descended into the gutter and now ranks alongside the Daily Mail as not just trivial but mendacious.
To be fair, the Times has also deteriorated. Now much more readable, it has frittered away its reputation for accuracy and can no longer be considered reliable. It’s instructive that its current editor, Tony Gallagher, is a former editor of the Daily Mail, the Telegraph and the Sun. Another valuable insight can be gained by reading the comments on the Telegraph and Times websites. They show a readership that is predominantly further to the right even than the publications themselves. This is alarming.
I now read both the Times and the the Guardian but I trust and respect the BBC’s journalism far more. I don’t understand why the BBC pays so much attention to the press. It is a dying medium, moving ever further to the authoritarian right on a daily basis. I would like to see the BBC stop following the press, stop allowing it to set the news agenda, stop reviewing the newspapers. Newspapers have nothing to offer the BBC and are a negative influence on its work.
Of course, I have my own issues with the BBC, its pro-Israel stance and failure to report fairly or accurately the Palestinian point of view. The Centre for Media Monitoring report of June 2025 analysed 35,000+ pieces of BBC content showing that Palestinian deaths are treated as less newsworthy. There is systematic language bias favouring Israelis and an almost complete suppression of genocide allegations with interviewees cut off as soon as they mention the word. Palestinian voices are suppressed with hardly any representatives given an opportunity to speak.
The other issue on which the BBC is failing badly is drugs policy. It simply isn’t covered While there are many reports of the ‘War on Drugs’, law enforcement activity, drug deaths, violence and gang warfare, never ever does the BBC look at policy. On any other issue, when a major problem is identified, there would be interviews with experts, analyses of policy options, etc. There is a complete blackout in the BBC on drugs policy. Some of this can be explained by the terrible truth that politicians don’t want to talk about it,. In fact they will do anything to evade the subject, only ever telling us that they are ‘tough on drugs’. There is a ‘group think’ in British politics and media that believes prohibition is the only option. They are too cowardly to look at the alternatives.
But I back the BBC. I want to see it toughen up. I want to see it do better on Israel and on drugs policy but overall no other broadcaster comes close. The people now attacking it: the Telegraph, the rest of the press, the Conservatives, Nigel Farage and Reform – well that just confirms it, I know exactly which side I’m on!
The Don Done Good

Trump’s two great skills have always been bragging and bullying. He’s used them both to great effect in Israel. Bullying was what Netanyahu needed and it was probably only Trump who could do it.
So while it does stick in my throat a little, I say thank you and pay him due respect for stopping the slaughter. I have grave fears that Netanyahu will yet find excuses to start the killing again but perhaps he will be in fear of upsetting Trump and will restrain himself.
Now the priority must be to maintain the peace but then to bring Netanyahu, some members of his government and the IDF to justice. This must also apply to some members of Hamas if they are still alive.
Providing the peace lasts, soon enough Trump’s attention will wander but the rest of the world must remain steadfast. If Netanyahu travels to any country other than the USA he must be arrested and sent to the Hague to stand trial.
What is Israel?

Israel is historical fiction. The idea comes from the Bible, the most successful work of fiction ever, and the story of a people called the Israelites, named after their ancestor who was called Israel or Jacob, the son of Isaac and grandson of Abraham. The legend developed and the name ‘Israel’ eventually came to refer to the people as a whole. The ‘Land of Israel’ is described as a promised homeland.
That is all there is. It has as much basis in fact as the Hobbit, the Loch Ness monster, Robin Hood or Beowulf.
Then in the 19th Century the idea of Zionism appeared driven by the need for a Jewish homeland in response to antisemitism, and influenced by European nationalism. Zionist leaders decided that there should be a Jewish homeland in Palestine. The idea developed over time, even gaining Hitler’s support in the 1930s, as he thought he could deport all German Jews to Palestine. Eventually after WWII and the Holocaust, the UK and USA supported the invasion of Palestine by about 100,000 Jewish refugees from Eastern Europe. They drove out about 750,000 Palestinians by military force and so established the state of Israel.
This was quite clearly a terrible war crime but to our eternal shame, probably because of guilt over the Holocaust, UK and USA manipulated the UN into post facto ‘legalisation’ of it. There you have the cause of all today’s problems after 77 years of slaughter and subjugation of Palestinians by Israel.
Charlie Kirk

I’d never heard of Charlie Kirk until he was shot, which was an awful, despicable crime. I’ve since learned that I profoundly disagree with him on almost everything but I am in awe of his courage and encouragement of open debate. It’s a sharp contrast with our weak, cowardly, mainstream politicians who don’t have the balls to debate difficult issues. Despite our differences, I think he was a hero and a martyr for democracy and free speech.
The Greater Israel Project. Netanyahu’s Real Agenda
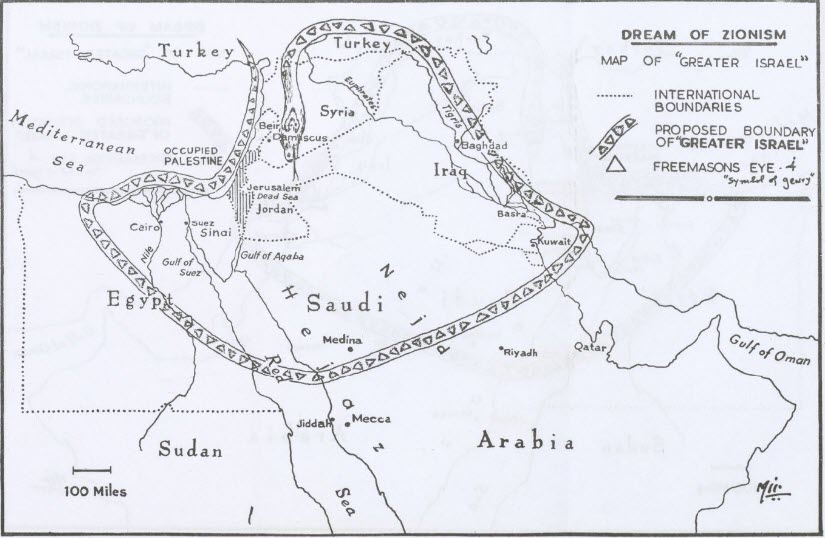
Netanyahu and his ultra-Zionist regime are pursuing the ‘Greater Israel’ project (Eretz Yisrael HaShlema). This claims the entire area from the River Nile, north-east to include the southern part of Turkey and south-east to include Iraq, Kuwait, west to include a large part of Saudi Arabia and about two-thirds of Egypt. This is based on the Zionist belief that around 2000 BC Abraham said ‘God’ had told him he and his descendants have the exclusive right to this land as defined in the Torah.
This includes annexation of half the Red Sea, the Suez Canal, the Sinai, Jordan, Gaza, the West Bank, Syria and Lebanon. Even the island of Cyprus is included in their ambitions. There is uproar in Cyprus right now from the native inhabitants as great tracts of land in the south of the island are being bought by Zioinist Israelis.
Ridiculous you may say and I would agree with you it seems so but Netanyahu has repeatedly shown his map of his ‘Greater Israel’ in public briefings confirming his government’s intent. Google it. You can see him holding a map with the area shaded in green.
There is also the ‘Ben Gurion Canal Project’ to cut a canal from Ashkelon, just north of Gaza, down to Eilat at the northern tip of the Red Sea. This would bypass the Suez Canal, just in case that ambition cannot be realised.
This is the reality of the ambition of the regime which is crazed with blood lust, expansionism and Zionist supremacy. Everything it is currently doing in Gaza and the West Bank is part of the process.

References
‘It’s Time to Confront Israel’s Version of “From the River to the Sea”’, The Nation, 22nd Nov 2023. https://www.thenation.com/article/world/its-time-to-confront-israels-version-of-from-the-river-to-the-sea/
‘What is the ‘Greater Israel’ movement?’, The Week, 18th Oct 2024. https://theweek.com/world-news/what-is-the-greater-israel-movement
‘Netanyahu says he’s on a ‘historic and spiritual mission,’ also feels a connection to vision of Greater Israel’, Times of Israel, 12th Aug 2025. https://www.timesofisrael.com/liveblog_entry/netanyahu-says-hes-on-a-historic-and-spiritual-mission-endorses-vision-of-greater-israel/
‘The “Greater Israel” Plan Has a Colossal Reach’, Fair Observer, 19th Dec 2024. https://www.fairobserver.com/politics/the-greater-israel-plan-has-a-colossal-reach/
‘Greater Israel—From the Euphrates to the Nile’, Times of Israel, 8th Jan 2024. https://blogs.timesofisrael.com/greater-israel-from-the-euphrates-to-the-nile/
‘Greater Israel’, Wikipedia. https://en.wikipedia.org/wiki/Greater_Israel
The Justification for Hiroshima and Nagasaki
Japanese disdain and disrespect for individual life was demonstrated by their treatment of prisoners of war and their suicidal attitude to military duty.
It was already clear by Iwo Jima that the Japanese strategy was to extract maximum casualties from their opposition in the certainty that they had no hope of victory. Data on casualties show the the US typically suffered three times as many wounded as killed but the Japanese literally fought to the death leaving very few wounded.
The two major battles before the atomic bomb was dropped were Iwo Jima and Okinawa.
Iwo Jima Deaths
US. 6,821
Japan. 17,845
Okinawa Deaths
US. 14,010
Japan. 77,823
Okinawan civilians. 149,634
US deaths for the invasion of Japan were estimated to be between 400,000 and 800,000. Japanese deaths were estimated to be over a million with civilian deaths of millions more.
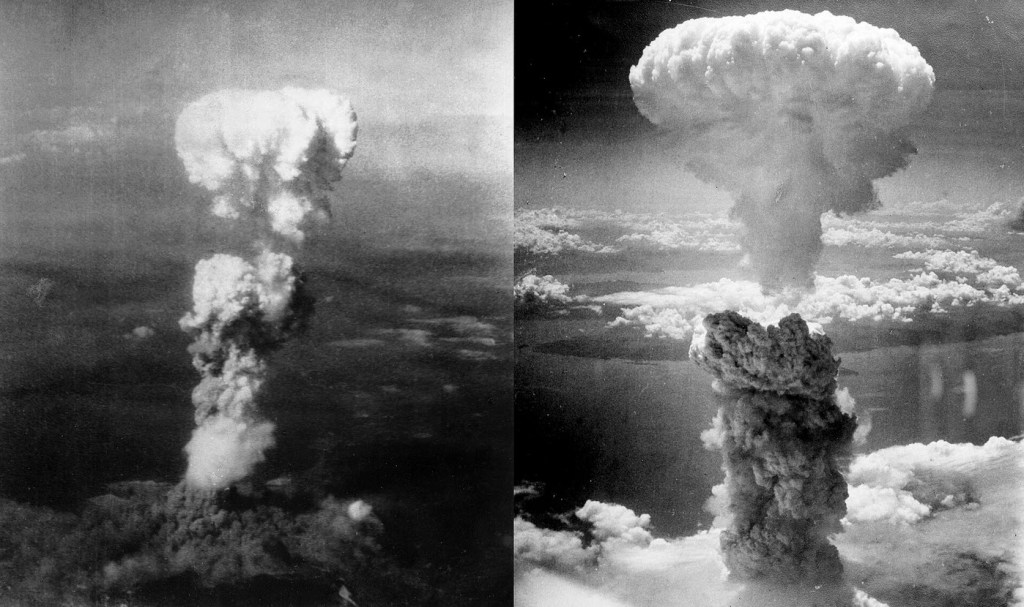
The Hiroshima bomb is estimated to have killed 135,000 including immediate and longer term deaths. The Nagasaki bomb caused a total of 50,000 deaths.
In my view the atomic bombs saved millions of lives and were justified.
Starmer Fiddles While Israel’s Genocide Gathers Pace
Possibly the best insight into what is really going on in Gaza is the video released yesterday from outside the areas where huge quantities of aid are stored waiting for the IDF to let it in.
It shows Israeli parents bringing their children to sit in the road to block lorries carrying aid to starving Palestinian children.
Israel is a perversion of humanity.
It is a moral imperative to recognise Palestine to advance its cause in the face of Israel’s genocide. But Starmer is a self-declared Zionist, a disciple of the death cult that prescribes death or forced displacement of native peoples to make way for Israel. He has repeatedly supported Israel’s war crimes, openly calling for it to cut off food and water to the besieged Gazan people. He provides surveillance flights over Gaza to assist the IDF with targeting civilians and he tacitly supports ‘settler’ terrorism in the West Bank.
Starmer’s future is clear. He will end his days in whichever prison cell the justices in the Hague send him.

The Future of the Land Between the River and the Sea
‘From the river to the sea, Palestine will be free!’ is a legitimate, non-exclusive ambition for freedom of the Palestinian people. It’s nothing like the exclusive, racist and hate filled Israeli slogan ‘Between the Sea and the Jordan there will only be Israeli sovereignty’, as enshrined in Netanyahu’s Likud Party constitution.
For all decent people, it makes no difference if you are Jewish, Muslim, Christian, Atheist, Israeli, Palestinian, British, American or a Buddhist from Outer Mongolia. To borrow from Martin Luther King, what matters is the “content of your character” and your actions.
The content of Israel’s ‘character’ and the history of its actions since its inception in 1948 are so disgusting, so barbaric that despite enormous support from the West, it has now descended into such sordid inhumanity that, unless it makes radical change, I don’t think it has any future.
Obviously a place is going to have be found for Israeli citizens, irrespective of whether they are Jews or not. The only solution that makes sense is the formal re-establishment of Palestine under supervision of the UN and the world community. Extraordinary safeguards will have to be put in place to protect the rights of all citizens, preserve their religious freedom and ensure there are no reprisals and no return to the sort of supremacist, apartheid society that Israel established.
Clearly this will not be an easy or trouble-free process but a line has to be drawn under the disaster of the past 77 years and the people of the region given a chance to live in peace, mutual respect and, in time, some sort of harmony.

