Posts Tagged ‘CBD’
My 11-Year Old Dog, Capone, Is A Miracle Of Medicinal Cannabis.
Capone Stanley Reynolds, to give him his full name, has been my faithful, handsome and sweet-natured companion since 2007. He really is a lovely dog, a strong silent type, very self-contained, gentle, calm and, I believe, wise.
Sadly, he developed epilepsy around the age of five and a couple of years later was struck with severe arthritis which means for the last three years or so he hasn’t been able to walk with me as he used to. However, regular use of CBD oil has transformed his life and I think we will have several more years together before he goes to that neverending walk in the sky where he will be able to run and play as he did when he was younger.
 He’s a cross between a Staffordshire Bull Terrier and a German Shorthaired Pointer – which is where he gets his gorgeous coat from, a mottled mixture of grey, black, white and a few touches of orange. I believe that, apart from his siblings, he is unique and he attracts a great deal of attention. People say he looks like a leopard and several times I have been offered large sums of money for him.
He’s a cross between a Staffordshire Bull Terrier and a German Shorthaired Pointer – which is where he gets his gorgeous coat from, a mottled mixture of grey, black, white and a few touches of orange. I believe that, apart from his siblings, he is unique and he attracts a great deal of attention. People say he looks like a leopard and several times I have been offered large sums of money for him.
We have walked hundreds of miles together. He first came to live with me when I lived in Emsworth, Hampshire. We learned the pleasure of walking together around Chichester Harbour and I had an article about our adventures published in Country Walking magazine.
I had once before, in the late 70s, seen someone fall down on a zebra crossing while having an epileptic fit. Nothing prepares you though for when someone you love first endures a seizure. It is frightening and deeply distressing. I can only despair at what it must be like for a parent whose small child suffers so.
Quickly though, you become used to it. You have to, for your own sake and so that you can look after the one who is fitting. In fact, there’s not a lot you can do, except protect them from hurting themselves while thrashing about. Every seizure is different but for Capone they all start with the most intense rigidity, arched back, teeth clenched and violent shaking. Then, after a minute or so, he will appear to relax and his legs will start a frantic bicycling motion while he froths at the mouth and usually loses control of his bladder, weeing everywhere. Occasionally he will go back into the rigid phase but at some point, usually within three or four minutes, he will jump slightly as if he’s just woken up – and indeed he has. Then he wants to stand up, although he doesn’t have proper control of his legs and he will fall over or walk into the wall or furniture. For up to an hour afterwards he will be wide-eyed, panting crazily and usually ravenously hungry. Gradually he calms down, until at last he sleeps, exhausted.
Capone’s seizures come in clusters over a 36 to 48 hour period. To begin with it was about every three hours, so it’s utterly draining, all through the night, never more than an hour or two’s sleep before the next one starts. When at last it comes to an end, it takes three or four days for him to recover. It’s almost like he’s had a stroke and he seems stupid, off balance and doesn’t really seem to know where he is. Thankfully, he always has recovered, right back to normal again and a week later it’s all forgotten.
I can’t remember the exact sequence of events now but it was around this time that the story of Charlotte Figi became known, the remarkable effect of CBD oil on this small child with Dravet’s Syndrome, a severe form of paediatric epilepsy. It wasn’t long before I decided to try Capone on CBD.
His arthritis had also dramatically worsened by now. We went from walking five miles every day to the point where it was taking the same amount of time for him just to walk half a mile or so. Both I and my other dog, Carla, were frustrated and suffering from a lack of exercise. Eventually I had to make the heartbreaking decision to leave him at home and just Carla and I would go for a walk. With a lack of exercise he began to put on weight and it became a vicious circle. About three years ago it had reached the stage where he couldn’t walk more than about 20 yards and I feared I would have to make the toughest decision of all. In this state, when a cluster of seizures came along, he truly was a pathetic sight, my wonderful, beautiful dog and friend in so much distress and pain.
I tried various CBD products. I didn’t really know what I was doing and they didn’t seem to have much impact. But then, nothing did. The best the vet could offer was rectal tubes of diazepam, like a small toothpaste tube with a nozzle that you stick up his bum and squeeze. They had no impact at all. I have given him 30mg of diazepam while he was fitting (enough to lay me flat out for 24 hours) and it’s made absolutely no difference. But then neither did CBD. There was none of this immediate effect like you see on the many YouTube videos of children being dosed with CBD oil.
 Gradually though the frequency and intensity of his seizures started to diminish. I had settled on using PlusCBD Gold oil. Two grams of this dissolved in olive or hempseed oil contains about 500mg of CBD and that would last for a month or so, giving him a dropper full every morning with his breakfast.
Gradually though the frequency and intensity of his seizures started to diminish. I had settled on using PlusCBD Gold oil. Two grams of this dissolved in olive or hempseed oil contains about 500mg of CBD and that would last for a month or so, giving him a dropper full every morning with his breakfast.
He was walking better. On a good day he could now manage a couple of hundred yards. In the summer he was able to do his very favourite thing and walk up the garden into full, unshaded sunlight and spend most of the day there sleeping on the lawn. The seizures seemed to have stopped.
Then, perhaps a year ago, I quadrupled his dose. I now use LoveHemp 20% oil which provides a full 2000mg of CBD. I dissolve this in olive or hempseed oil in a 50ml dropper bottle and he continues to get one dropper full every day.
olive or hempseed oil in a 50ml dropper bottle and he continues to get one dropper full every day.
In the past two years, Capone has had just one cluster of seizures. It took place over the same period but there were far fewer fits of much less intensity, perhaps seven or eight over 48 hours. He can walk a few hundred yards now. He’ll never be the vigorous, fast-running dog he once was but occasionally I take him for a slow walk now for half an hour or so. If he sees another dog he gets excited and gets up a rather ungainly and clumsy turn of pace – but it’s almost a run and he’s still Capone and I treasure every minute that we have together. CBD oil, or as it should be more accurately termed, low-THC whole plant cannabis extract, has saved his life.
MHRA Backtracking Super Fast On CBD Ban.
 In advance of the meeting between the UK Cannabis Trade Association (UKCTA) and the MHRA on Thursday, there has been a flurry of activity which amounts to a climb down by the regulator.
In advance of the meeting between the UK Cannabis Trade Association (UKCTA) and the MHRA on Thursday, there has been a flurry of activity which amounts to a climb down by the regulator.
This statement was published on the MHRA website at lunchtime today.
“Update 1 November 2016
An MHRA spokesperson said:
While MHRA has given its opinion that products containing cannabidiol (CBD) used for medical purposes are medicines, we have also carefully considered the needs of individuals using CBD products to treat or manage the symptoms of medical conditions.
Our primary concern is patient safety. In order to ensure that products remain available until individuals have the opportunity to discuss their treatment with their doctor, companies now have until 31 December 2016 to voluntarily operate within the law, by withdrawing their existing products from the market, or working with MHRA to satisfy the legal requirements of the Human Medicines Regulations 2012.
We have today written to the manufacturers of CBD to make them aware of the timeline for engagement.
It is vital that medicines meet safety, quality and efficacy standards to protect public health.”
Originally the MHRA wrote to CBD suppliers in threatening terms:
“You must cease to sell, supply, promote, advertise or process orders for the above products until appropriate authorisation has been granted for them. You must confirm this in writing within 28 days from the date of this letter that you have taken the above steps.”
So quite a change in tone. The MHRA seems to have recognised that contrary to its declared mission ‘to improve health’, its original statement actually endangered the health of tens of thousands of people.
Additionally, solicitors representing the UKCTA have now written to the MHRA seeking clarification of its intentions and making three crucial points:
- The letters to CBD suppliers and the MHRA’s original press statement have caused serious financial damage to the CBD industry.
- The MHRA has conducted no effective consultation with stakeholders.
- The MHRA’s own guidelines require it to consider each product on a case by case basis and a blanket ban on products containing CBD would be unlawful.
Professor Mike Barnes, scientific and medical advisor to CLEAR, commented:
“The MHRA’s new stance is an improvement from their previous position. However, I cannot see any value in delaying only a few months. Some patients might be able to find an alternative medicine from their doctor but many people will have already tried alternative medications and found that CBD is the only satisfactory treatment for their condition. This is the case, for example, for children with epilepsy who will have almost certainly have been under the care of a specialist and tried available anticonvulsants and found that CBD is the only treatment that works for them. The MHRA does not seem to realise the impact of this arbitrary and rushed decision which will clearly be detrimental and potentially have very serious (and in some cases life threatening) implications for some people. The MHRA need to work with the manufacturers and the medical profession to determine the best way forward that both recognises that cannabis based products have medicinal value, and as such need proper trials of efficacy and safety, yet on the other hand does not place existing users at risk of harm”.
UK Cannabis Trade Association Meeting With MHRA This Week.
After all the speculation, many misleading and false reports and a plethora of attempts to interpret the MHRA’s actions concerning cannabidiol (CBD), this week the chips are down.
On Thursday 3rd November, at MHRA headquarters in Victoria, six representatives of the UK Cannabis Trade Association (UKCTA) will sit down with those responsible for the agency’s statements on CBD. We will be armed with counsel’s opinion on the legality of the MHRA’s action but most importantly we hope to secure clarification for those who rely on CBD as a food supplement. We will publish details of the outcome of the meeting as soon as we can.
Those attending as UKCTA representatives are:
Anthony Cohen, Elixinol UK
Mike Harlington, GroGlo Horticultural Research & Development
Peter Reynolds, CLEAR Cannabis Law Reform
Tom Rowland, CBD Oils UK
Karl Spratt, Hempire
Tom Whettem, Canabidol
MHRA Confirms Meeting With CBD Industry Representatives.
Today, the Medicines and Healthcare products Regulatory Agency (MHRA), has arranged a meeting with representatives from the UK Cannabis Trade Association (UKCTA) to discuss its designation of cannabidiol (CBD) as a medicine.
 A request for a meeting was was first made in writing on 20th September 2016, when the possibility of the MHRA’s action was still little more than a rumour. Nearly six weeks later, after repeated requests, complaints and lobbying from many companies, individuals and MPs, the meeting has been fixed for 3rd November 2016.
A request for a meeting was was first made in writing on 20th September 2016, when the possibility of the MHRA’s action was still little more than a rumour. Nearly six weeks later, after repeated requests, complaints and lobbying from many companies, individuals and MPs, the meeting has been fixed for 3rd November 2016.
The main aim of the meeting will be to discuss interim arrangements for people currently using CBD as a food supplement. Clearly, we will also address concerns over the impact of this decision on many small businesses and the people they employ.
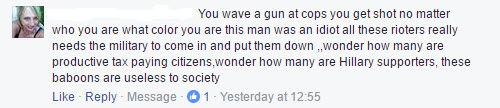
Facebook Says Calling A Black Man A Baboon “Doesn’t Violate Our Community Standards”.
On the other hand Facebook says that recommending a responsible, reputable supplier of verified, lab-tested, legal CBD food supplements does violate its standards.
At a guess (because you can’t get a straight answer from Facebook about anything), the issue is “We prohibit any attempts by unauthorised dealers to purchase, sell or trade prescription drugs, marijuana, firearms or ammunition.”
Now CBD food supplements are fully legal products. They are not prescription drugs. True, CBD is present in cannabis but it is also found in many other plants. So it’s difficult to understand what the problem is – but not as difficult as getting a coherent answer from Mr Zuckerberg and his disciples.
For the ‘offence’ of recommending a CBD supplier your page gets a seriously heavy warning to all page admins, a threat of permanent deletion and I, as the author of the post sharing a link to CBD Oils UK, was banned from Facebook for 30 days. Such is the reality of living under the diktat of the unaccountable, overbearing, bureaucratic monolith that Facebook has become.
However, when some vile American Trumpoid leaves a comment on the CLEAR page calling a black man a baboon, that’s just fine and dandy.
It is time that Facebook was placed under serious regulation for its unfair and oppressive trading practices. It has become so ubiquitous that it now has a responsibility that goes beyond any independent business. It is virtually impossible for individuals and small businesses to operate without a Facebook account. It should be subject to strict standards and forced to comply with fair practices.
I’m all for free enterprise but it’s time to slam Facebook hard for its tax dodging, its failure to take responsibility for publishing abuse and its unfair treatment of users and advertisers.