Cruel And Irresponsible Response from UK Government To Parliamentary Report On Medicinal Cannabis.
Unsurprisingly perhaps, the response to the recent call from MPs and peers to legalise cannabis for medicinal use has come straight from the top. Theresa May’s longstanding reputation as a denier of science and evidence on drugs policy is reinforced by her peremptory dismissal of the expert report. It seems that, at least in the short term, the UK government is sticking by a policy that is discredited, ridiculous and deeply cruel.
It fell to Sarah Newton MP, minister of state at the Home Office, to respond to a parliamentary question from Roger Godsiff, Labour MP for Birmingham, Hall Green.
“To ask the Secretary of State for the Home Department, if she will respond to the recommendations of the report by the All-Party Parliamentary Group for Drug Policy Reform Accessing Medicinal Cannabis: Meeting Patients’ Needs, published in September 2016.”
“The Prime Minister responded to the All-Party Parliamentary Group for Drug Policy Reform’s report ‘Accessing Medicinal Cannabis: Meeting Patients’ Needs’ on the 27 October.
Cannabis is controlled as a Class B drug under the Misuse of Drugs Act 1971 and, in its raw form, currently has no recognised medicinal benefits in the UK. It is therefore listed as a Schedule 1 drug under the Misuse of Drugs Regulations 2001.
It is important that all medicines containing controlled drugs are thoroughly trialled to ensure they meet rigorous standards so that doctors and patients are sure of their efficacy and safety. To do otherwise for cannabis would amount to a circumvention of the clearly established and necessary regime for approving medicines in the UK.”
In other words, this is nothing more than a re-statement of the same position that the UK government has held since 1971 when legal access to medicinal cannabis was halted. Quite clearly the government has given no consideration at all to the vast amount of scientific evidence and international experience that has accumulated over the last 45 years. The latest report which took nine months to produce, took evidence from over 600 witnesses and included a review of over 20,000 scientific studies is simply cast aside. To be honest, I doubt whether it has even been read by Ms May or anyone in the Home Office or Department of Health. This is the standard that now prevails in the UK – government of the people by an unaccountable, out-of-touch, unresponsive cabal of individuals elected by a deeply flawed system that gives democracy a bad name.
On the face of it, the claim that all medicines must be thoroughly trialled seems plausible – but it is not. It is a misleading half-truth clearly intended to squash the call for access to medicinal cannabis by painting a false picture.
Doctors are allowed to prescribe any medicine, licensed or unlicensed, as they see fit, based on their own judgement. But prescribing of cannabis is specifically prohibited by Statutory Instrument despite the scientific consensus that it is far less dangerous than many, probably most commonly prescribed medicines.
So it’s not a level playing field. It’s a policy that is based on prejudice and scaremongering about recreational use of cannabis. Ms Newton’s answer is at best disingenuous but then she probably doesn’t even realise that herself. For many years Home Office policy has been systematically to mislead and misinform on cannabis and evidently under Ms May’s successor, Amber Rudd MP, such dishonesty continues.
Something will eventually force the government’s hand to change its absurd position on cannabis. Sadly the very last consideration will be scientific evidence or the will of the people. Such factors hold no sway with UK governments. Only when enough of the political elite open their eyes and examine their conscience, or some key individuals or their family members, experience the need for medicinal cannabis will change become possible. Alternatively, political upheaval may present an opportunity. The Liberal Democrats were too cowardly, weak and concerned with building their personal careers when in coalition to advance the cause they now so bravely advocate. Perhaps the SNP, with 56 MPs, all in favour of medicinal cannabis may be our best hope.
Sarah Newton is merely a puppet of the Home Office bureaucracy. Theresa May’s mendacious position on all aspects of drugs policy is well established and she is as stubborn and bigoted as they come on such matters. Only when she, in person, is subject to sufficient pressure will this cruel, ignorant and hateful policy change.
Wise Words From Trump Supporters?
Some thoughts here that chitter-chattering, Remoaners, Remaniacs, the soft left and particularly the illiberal, anti-democratic LibDems would do well to consider.
How did this happen you ask?
You created “us” when you attacked our freedom of speech.
You created “us” when you attacked our right to bear arms.
You created “us” when you attacked our Christian beliefs.
You created “us” when you constantly referred to us as racists.
You created “us” when you constantly called us xenophobic.
You created “us” when you told us to get on board or get out of the way.
You created “us” when you forced us to buy health care and then financially penalized us for not participating.
You created “us” when you allowed our jobs to continue to leave our country.
You created “us” when you attacked our flag.
You created “us” when you confused women’s rights with feminism.
You created “us” when you began to emasculate men.
You created “us” when you decided to make our children soft.
You created “us” when you decided to vote for progressive ideals.
You created “us” when you attacked our way of life.
You created “us” when you decided to let our government get out of control.
“You” created “us” the silent majority.
And we became fed up and we pushed back and spoke up.
And we did it with ballots, not bullets.
I Am Not A Badge Of Honour.
I am not a badge of honour,
I am not a racist smear,
I am not a fashion statement,
To be worn but once a year,
I am not glorification
Of conflict or of war.
I am not a paper ornament
A token,
I am more.
I am a loving memory,
Of a father or a son,
A permanent reminder
Of each and every one.
I’m paper or enamel
I’m old or shining new,
I’m a way of saying thank you,
To every one of you.
I am a simple poppy
A Reminder to you all,
That courage faith and honour,
Will stand where heroes fall.
CLEAR Statement Concerning Cannabis Legalisation Measures In US Election.
“This is marvellous news for liberty, health and human rights. The USA, unlike Britain, has a functioning democracy where the will of the people prevails rather than the bigotry and self-interest of politicians. It is wonderful to see that truth, justice and evidence is winning out over the lies and misinformation we have been fed about cannabis for almost 100 years.
In 1971, the British government abdicated all responsibility on cannabis and abandoned our communities and our children to criminal gangs. Since then all the harms have multiplied exponentially. The laws against cannabis fund organised crime, promote dangerous hidden farms which are fire risks, the destruction of rental property, selling to children, contaminated ‘moonshine’ cannabis, gang violence, lives ruined by criminal records and the cruel denial of safe, effective medicine that can relieve pain, suffering and disability.
Donald Trump has supported access to medicinal cannabis all along. Many British politicians who consider him to be an unreasonable person should now look to themselves and ask whether they are being reasonable by supporting prohibition, even for medical use.
It is time for Theresa May, Amber Rudd and the UK government to take responsibility for the £6 billion pa cannabis market. The tide of legalisation is now unstoppable and it would be deeply irresponsible for them to fail to act. They must grasp this nettle now!”
Peter Reynolds, president of CLEAR Cannabis Law Reform
CLEAR Cannabis Law Reform Accounts 2015.
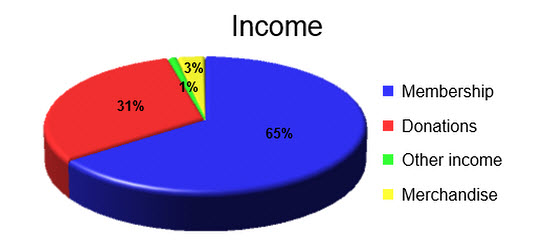
Income
Compared to the previous year, CLEAR’s regular income in 2015 was up 79% to £17,074. The majority of income continues to come from memberships, with the remainder coming from donations, merchandise and Google advertising.
Regular income: £17,074
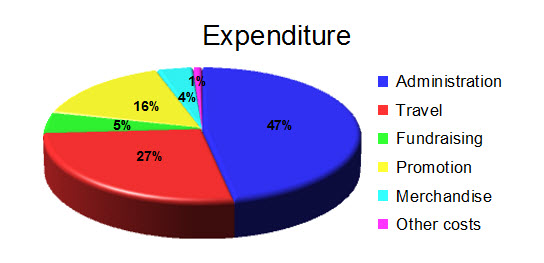
Expenditure
CLEAR spent a total of £12,023, a decrease of 11% on the previous year.
Total expenditure: £12,023
Administration: membership administration, stationery, postage, telephone & internet, meeting expenses, etc. Administration costs have increased as an overall proportion of expenditure as there were no dedicated campaigns during the year.
Travel: expenses incurred meeting government ministers, MPs, agency representatives, media engagements, boards meetings, also re-imbursement of travel costs for Medicinal Use Panel members
Fundraising costs: PayPal fees and other fundraising costs
Promotion: Facebook advertising, printing of leaflets, design work, etc.
Breakfast Of Champions 2.
A recent invention of mine.
Bake the black pudding at about 150 C for about 10 minutes. Chop roughly and fry in butter for a few moments in the omelette pan to give crispy edges. Add as many well-seasoned, beaten eggs as you wish and cook for a couple of minutes. You can fold it or serve it flat and cut into sections.
Remember to give the dogs a little taste, it’s only fair.
Tip. Please keep the inside of the omelette as runny as you can, it makes all the difference. After decades of experience as a black pudding connoisseur, I buy mine from Framptons of Bridport. It’s described as ‘local’ but it isn’t that local because it’s made in Poole – but it is the business!